Danger! Caution! Inhaling carbon dust
into the lungs can cause lung cancer.
https://www.0ho.co.jp/carbon_list/
Introduction to carbon
1. Difference between carbon and graphite - difference between carbon and graphite
Graphite is written as graphite in kanji, but the Japanese name is graphite.
The "lead" in the name is because before the elemental analysis of graphite, it was thought to contain lead. It was originally called (plumbago) in Latin, and (black lead) in English. I literally translated it as "graphite". The name graphite was given after the element was discovered to be "C" (carbon).
Now, let me briefly explain the difference between carbon and graphite.
What is Carbon?
It is represented by the atomic number 6 and the element symbol "C". It exists as a compound in which the carbon atom "C" and other substances such as coal, petroleum, and asphalt are combined, or as a mass of pure "C" atoms such as graphite and diamond. .
Organic compounds (organic substances) are the general term for compounds that have the atom "C" as the skeleton of the structure. Think of carbon as a substance (element) that forms the basis of organic matter (organic compounds).
However, although it is an excellent organic substance, allotropes such as "graphite" and "diamond", which are aggregates of pure carbon atoms, monoxide/carbon dioxide, metal carbonates (calcium carbonate, etc.) and metal cyanates/metals Thiocyanate is considered an inorganic compound (inorganic) while having the atom "C" in its structural backbone. This is because in the past, organic compounds were defined as “chemical substances produced by living organisms.” Carbon compounds, which were discovered prior to this definition as chemical compounds not related to living organisms, were inorganic compounds (inorganic substances). ).
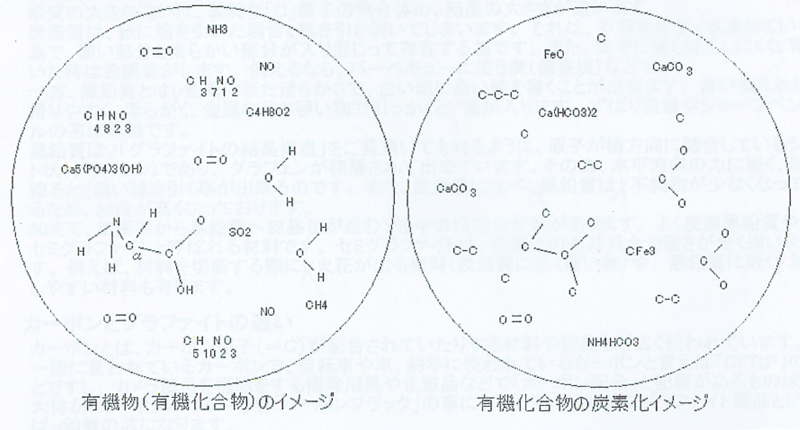
What is carbonization
Organic compounds (organic substances), which are carbon compounds, become ash or charcoal by heating them in an inert atmosphere (for example, a gas atmosphere such as nitrogen or argon) or in a sealed air environment at several hundred degrees to 1,000 degrees. Carbonization is the process of heating an organic compound (organic matter) to turn it into ash or charcoal.
In the natural world, ancient dinosaurs and plants are buried in the ground and are affected by geothermal heat and ground pressure in a state where the air is shut off. .
An easy-to-understand example of charcoal produced by artificial methods is charcoal used for barbecues.
Charcoal is steamed in a pot without exposing the wood to air. The process of turning wood into charcoal is called carbonization.
It is a state in which organic compounds (organic matter) that have turned into "charcoal" in the range of several hundred degrees to 1,000 degrees, small substances with the crystal structure of graphite or diamond, and other substances (impurities) are included. Substances that do not have a fixed crystal structure are called "amorphous carbon".
Most of the organic compounds (organics) are transformed into various types of amorphous carbon by the addition of heat. For example, various resources are made from primitive dinosaurs, trees and living things. Solid (solid phase) substances such as coal, coke, charcoal, and soot; liquid (liquid phase) substances such as naphtha (gasoline), kerosene, light oil, and heavy oil; gas phase).
*The three states of matter (solid, liquid, gas) are called "three states of matter" or "three phases".
What is graphitization
The charcoal is heated at a temperature as high as 2,700 to 3,000°C while the air is shut off. By heat-treating (adding heat), it is possible to burn and vaporize the impurities around the "C" atom. This high temperature heat treatment is called “graphitization”.
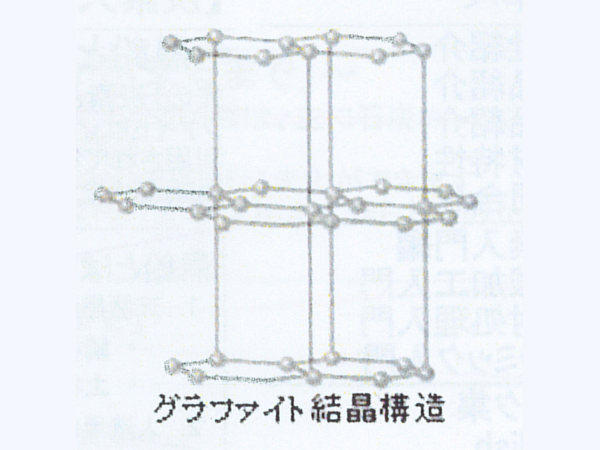
Pure charcoal made by graphitization (crystals of "C" atoms in a regular array) is called graphite. Graphite atoms are hexagonal plate crystals. The structure is a tortoiseshell-like layered substance.
What is graphite?
It refers to a lump of carbon with a regular arrangement that is produced by high-temperature heat treatment called graphitization. In the natural world, if you continue to apply high-temperature heat to carbonized carbon, it will become "natural graphite". On the other hand, graphite that is made by manipulating charcoal at high temperature is called "artificial graphite".
Whether natural or man-made, if you continue to apply heat and tremendous pressure to graphite, it will turn into diamond. The natural diamond you are wearing is a precious and mysterious thing that was heat-treated deep in the ground and appeared near the ground due to crustal movements.
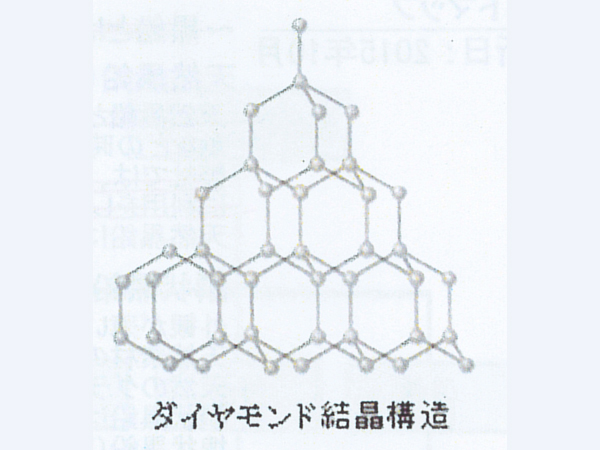
Difference between carbonaceous and graphite
Carbonaceous refers to those with a low degree of crystal development that are formed only by the atom "C". The major difference between graphite and carbonaceous matter is the size of crystals of aggregates of pure "C" atoms.
Carbonaceous will tear the paper if you draw a streak on it. This is because it contains a lot of impurities, and it has a mixture of hard and soft parts. In addition, it is very hard and difficult to process, and when struck, it makes a metallic sound. For example, charcoal used for barbecue (Bincho charcoal).
Graphite, on the other hand, has a smoothness similar to that of a pencil and can be used to draw black lines on white paper. In other words, it is slippery, soft, and scratched by hard objects such as metal. Leads of straight pencils and mechanical pencils are graphite.
As you can see from the "Crystal structure of graphite", graphite is a sheet (graphene) in which atoms are bonded in the horizontal direction, and is made up of layers of graphene. Therefore, it is weak against horizontal force and can draw a black line when rubbed against paper. In addition, graphite has less impurities than carbon, so it has a higher purity.
In addition, there are intermediate stage materials that crystallize from carbonaceous to graphite. This material is often called carbon-graphite or semi-graphite. Semi-graphite has a completely different hardness depending on the degree of crystallization. For example, there are materials that generate sparks when cut (hard materials that are close to carbonaceous) and materials that are easy to process because they are close to graphite.
Difference Between Carbon and Graphite
Carbon is widely used in materials and products containing carbon atoms (=C). Generally speaking, carbon used in bicycles, cars, and fishing rods is “CFRP”. Cleaning tools and cosmetics for cleaning camera parts that are described as "carbon-containing" are mostly "carbon powder" or "carbon black". A common graphite product is the lead of a pencil.
Industrial carbon often refers to graphite, and it is used as a jig for making something, as a part of machine parts, and as a raw material for industrial products. increase. There are not many people who use graphite and carbon separately.
For this reason, I think the correct way to use carbon is to refer to "carbon materials in general" and graphite refers to "carbon materials that are graphitized".
Carbon in industrial applications is mainly referred to as graphite.
Introduction to Carbon List
- 1. Difference between carbon and graphite - difference between carbon and graphite
- 2. What is graphite? (Types of graphite) "Natural graphite and artificial graphite"
- 3.Manufacturing method of artificial graphite "Until the graphite block is made"
- 4.Introduction to graphite materials (differences in characteristics depending on the manufacturing method of artificial graphite materials)
- 5. Familiar carbon products, carbon products
- 6. There are graphite products in such places! ?
- 7. What will the carbon of the future look like? (New carbon/Fine carbon)
- 8.What are the properties of carbon?
Consultation and quotation for carbon products
Business hours Weekdays 8:30-17:30 (excluding weekends and holidays)
Introduction to carbon
https://www.0ho.co.jp/carbon/p7/
7. What will carbon be like in the future? (New carbon/Fine carbon)
Carbon has been commercialized in various ways even at the atomic level. Carbon is considered to be important in making future life better, and various studies are constantly being carried out.
- 1 Carbon nanotube (=Carbon Nano Tube)
- 2 Carbon Nano Horn
- 3 fullerene
- Four Carbon Nano Capsules
- Five fullerene polymer
- 6 lonsday light
- 7 amorphous carbon
- 8 nanocrystalline diamond film
- 9 nanotube coat
- Ten ultra thin tv
- 11 High performance rice husk activated carbon
- 12 Super poly fullerene hydroxide
- 13 carbon nanotube actuator
- 14 Fuel cells for mobile devices
- 15 Automotive fuel cell
- 16 Composite structure of carbon nanotubes and graphene
- 17 CFRP artificial joints/prostheses
- 18 carbon nano gauge
Carbon nanotube (=Carbon Nano Tube)
 Carbon nanotubes are carbon atoms bonded together in a cylindrical shape. It has high current density resistance, high thermal conductivity, high mechanical strength, and an elongated molecular structure. It is said that by bundling unbroken nanotubes into a 1 cm length, it is possible to lift a weight of 1200 tons. Carbon nanotubes are called "CNT".
Carbon nanotubes are carbon atoms bonded together in a cylindrical shape. It has high current density resistance, high thermal conductivity, high mechanical strength, and an elongated molecular structure. It is said that by bundling unbroken nanotubes into a 1 cm length, it is possible to lift a weight of 1200 tons. Carbon nanotubes are called "CNT".
Carbon Nano Horn
A carbon nanotube with a closed horn tip.
When the carbon nanohorn complex was used to irradiate mouse tumors with laser light, a remarkable therapeutic effect was confirmed. In the future, if the research progresses, it will greatly contribute to the cancer treatment of the human body.
fullerene
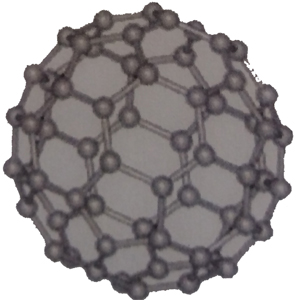 A molecule called fullerene C60, which has a soccer ball-like structure and is composed of 60 carbon atoms, has attracted particular attention. This has been put to practical use as a high lubricating material (friction coefficient is almost 0). In addition, fullerene has been shown to cleave intracellular DNA, eliminate active oxygen, and have anti-HIV activity by the action of light, and clinical trials for medical use are being conducted.
A molecule called fullerene C60, which has a soccer ball-like structure and is composed of 60 carbon atoms, has attracted particular attention. This has been put to practical use as a high lubricating material (friction coefficient is almost 0). In addition, fullerene has been shown to cleave intracellular DNA, eliminate active oxygen, and have anti-HIV activity by the action of light, and clinical trials for medical use are being conducted.
Carbon Nano Capsules
In the research process of fullerene production, a technology has been developed to enclose metal ultrafine particles in fullerene spherical bonds. This is excellent in airtightness, protects against oxidation and water decomposition while drawing out the properties of the metal inside, and research is progressing on technology that utilizes the lubricity of graphite.
fullerene polymer
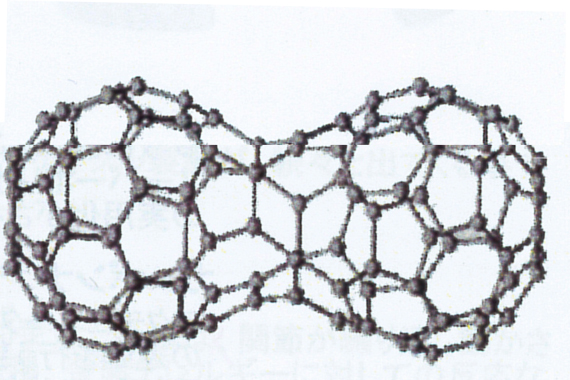 Research is being conducted on "fullerene polymers" that bind fullerenes together.
Research is being conducted on "fullerene polymers" that bind fullerenes together.
In fullerene polymers, one fullerene bond serves as one carbon atom, and there are various structures such as hexahedral bonds similar to graphite bonds, peanut-shaped doubles, and straight connections. An interesting function that appears for the first time in carbon as a fullerene polymer is to have "magnetism". The cause of magnetism has not been elucidated. Research on fullerene polymers, such as whether they have other properties, is still underway.
lonsday light
It is a crystal of the same carbon atoms as diamond, and is also called "Hexagonal diamond" because of its crystal structure.
The actual Mohs hardness of lonsdaleite is about 3 to 7 due to possible factors such as impurities contained in the material and bonding defects, but pure lonsdaleite can withstand 58% higher pressure than diamond. is expected. This is because it is a substance created by the collision of meteorites, and it is confirmed in minute amounts in meteorites, and even if it is large, it is small enough to be confirmed with a microscope. This is because there is no lonsdaleite, which is a large crystal of pure bonding, and it cannot be measured.
Future industrial applications are being explored for use in drill bit tips and spacecraft, and artificial production methods for lonsdaleite will also be explored in the future.
amorphous carbon
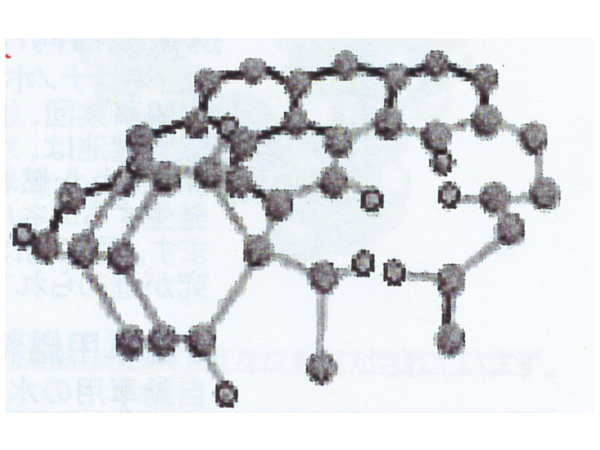 Carbon does not have a fixed shape such as spherical or planar, and does not have a fixed pattern of molecular bonds in a state where carbon and hydrogen (H) are combined. In other words, it does not have a fixed bonding structure like diamond (SP3 structure) or graphite (SP2 structure).
Carbon does not have a fixed shape such as spherical or planar, and does not have a fixed pattern of molecular bonds in a state where carbon and hydrogen (H) are combined. In other words, it does not have a fixed bonding structure like diamond (SP3 structure) or graphite (SP2 structure).
Amorphous carbon:
i. ii
. Good abrasion resistance
iii. Chemically stable
iv. Hard
v. A flat film can be obtained
vi. Low Young's modulus
vii. It generally
has the property of being an insulator. Amorphous carbon is used as a material for coating various things.
Applications include plasma etcher electrode plates, dummy wafers, and fuel cell separators in the electronics and semiconductor fields. In addition, crucibles and pipes are coated with a film for their chemical resistance, high purity, and thermal shock resistance. Because of its corrosion resistance and heat resistance, it is used in semiconductor manufacturing jigs.
nanocrystalline diamond film
Nanocrystalline diamond film (UNCD) is a film made of nano-sized pure diamond crystals.
Since it is a nano-sized film, it can be attached to various objects and its strength can be increased by coating it.
Applying a nanocrystalline diamond film reduces friction, increases wear resistance, and reduces heat generation, making it possible to save energy.
nanotube coat
A research group led by Prof. Katsuyoshi Kondo and Associate Prof. Junko Umeda of Osaka University has developed a technology to efficiently coat carbon nanotubes on metal surfaces.
Applying a nanotube coat to the metal makes it durable and makes it possible to suppress the friction of the metal. It is possible to coat a wide range of materials such as titanium, aluminum, and iron, and it is expected to be applied to machine parts such as automobile and airplane engines, transmissions, and bearings, as well as home appliances.
ultra thin tv
As for the application of carbon nanotubes, NEC and Ise Electronics have announced surprisingly thin nanotube display prototypes. Future televisions may be wall-mounted at 65 inches, 5mm thick, and may consume a third of the current power consumption.
High performance rice husk activated carbon
Rice husks were used for feed and bedding, and most of them were disposed of by open burning. Incineration of rice requires a large amount of oxygen. Therefore, the emission of greenhouse gases due to carbon dioxide has become a problem. The charred rice husk contains silicon dioxide, and there was a problem that it was difficult to industrially recycle it for a wide range of purposes.
A research group at Nagaoka University of Technology succeeded in removing silicon dioxide from rice husks by heat treatment using potassium hydroxide and sodium hydroxide.
Conventional activated carbon has a surface area of 1,000 square meters per gram, but it has been discovered that high-performance rice husk activated carbon has a surface area about 2.5 times larger and has high adsorption power. Therefore, various applications are being considered, such as using it as a fuel cell material that adsorbs a large amount of hydrogen.
Super poly fullerene hydroxide
In joint research with Vitamin C60 Bioresearch, a nanotechnology subsidiary of Mitsubishi Corporation, and the research group of Professor Takumi Oshima of the Department of Materials Chemistry, Graduate School of Engineering, Osaka University, a large number of hydroxyl groups (OH-) are modified on the carbon atoms of fullerenes. Water-soluble "super poly fullerene hydroxide" has been developed. This super polyhydroxide fullerene efficiently eliminates active oxygen harmful to the human body, so it has been put to practical use in cosmetics.
carbon nanotube actuator
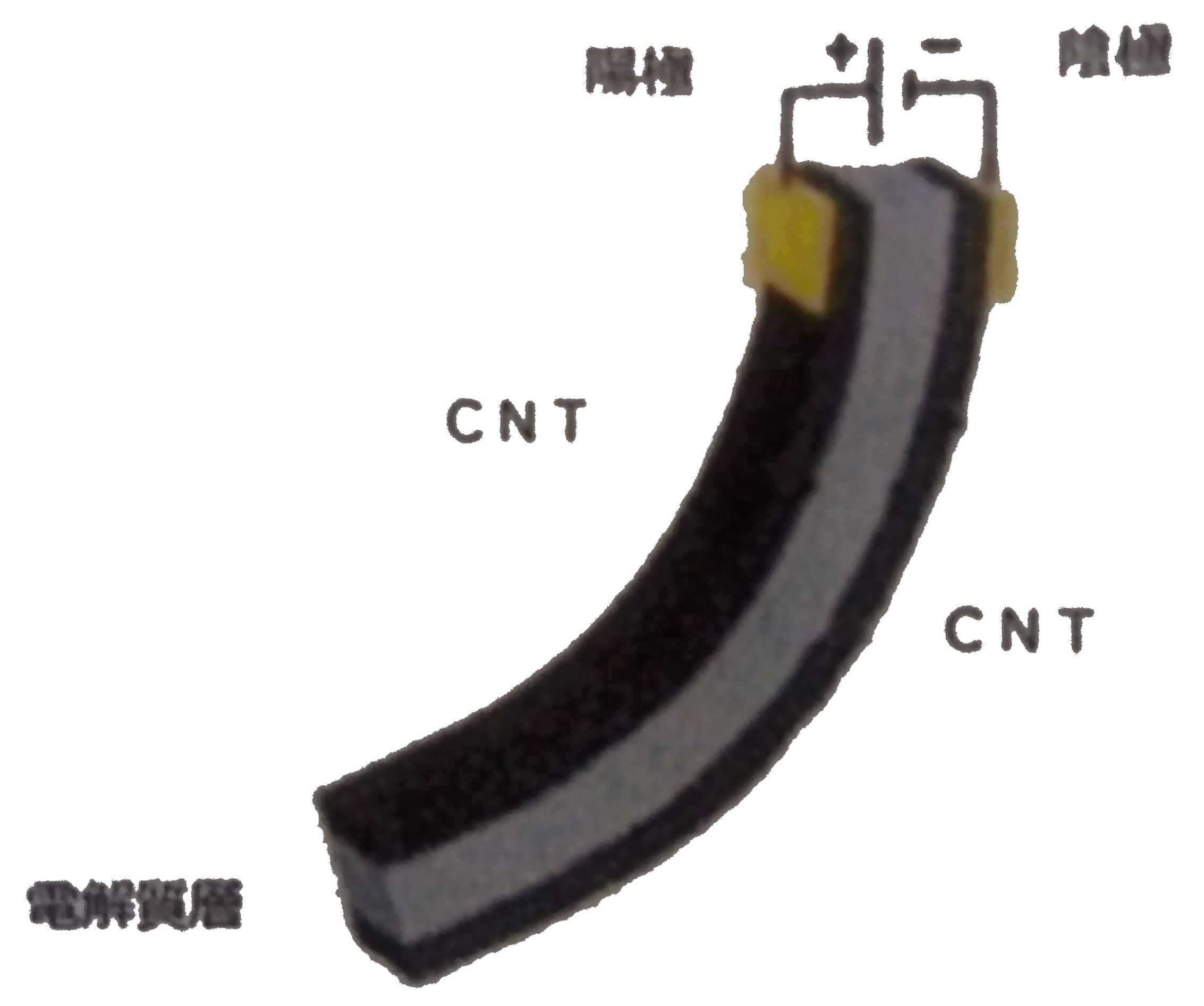 An actuator is a device that performs movement such as stretching and bending by sending a signal.
An actuator is a device that performs movement such as stretching and bending by sending a signal.
Actuators are often used in home appliances and aircraft. For example, Christmas lights, car turn signals, etc. When electricity is passed through, the switch automatically turns on and off due to the thermal expansion of the copper electrode. It would be easy to understand if it is a device that generates a similar movement by a signal.
Using carbon nanotubes for these electrodes, research into ultra-compact actuators is underway, and research into joint movements and artificial muscles in his robot is underway.
The other day, through joint development by the artificial cell research group of the National Institute of Advanced Industrial Science and Technology Health Engineering Research Institute and Alps Giken Sendai Development Center, Super Growth Carbon developed by the Nanotube Application Research Center of the National Institute of Advanced Industrial Science and Technology Using a nanotube (SG-CNT), the displacement is reduced by only 10% even if it is driven 100,000 times.It is highly durable and has a displacement retention property that can keep the displacement state almost constant for 3 hours. A high-performance nanocarbon polymer actuator was developed. The results of this research will likely lead to further development for practical application.
Fuel cells for mobile devices
Small fuel cells for mobile devices that use carbon nanohorns as electrodes have been announced by NEC Corporation, the Japan Science and Technology Agency, and the Institute of Industrial Creation. Fuel cells generate electricity by reacting a fuel such as hydrogen with oxygen.
It has been confirmed that the developed fuel cell has an electrical output that is approximately 20% higher than before. Furthermore, the energy generated is about 10 times that of lithium batteries, and it is attracting attention as a highly efficient next-generation energy source. In the future, it is said that it will be possible to use notebook computers continuously for several days, and further research is underway.
Automotive fuel cell
A particular problem in the development of hydrogen fuel cells for automobiles is how to make hydrogen fuel tanks that are safe, lightweight and small.
Although it is still in the research stage, research is underway to coat the fuel tank with metallic titanium, fill the tank with carbon nanotubes, adsorb hydrogen to the nanotubes, and store it as fuel.
Composite structure of carbon nanotubes and graphene
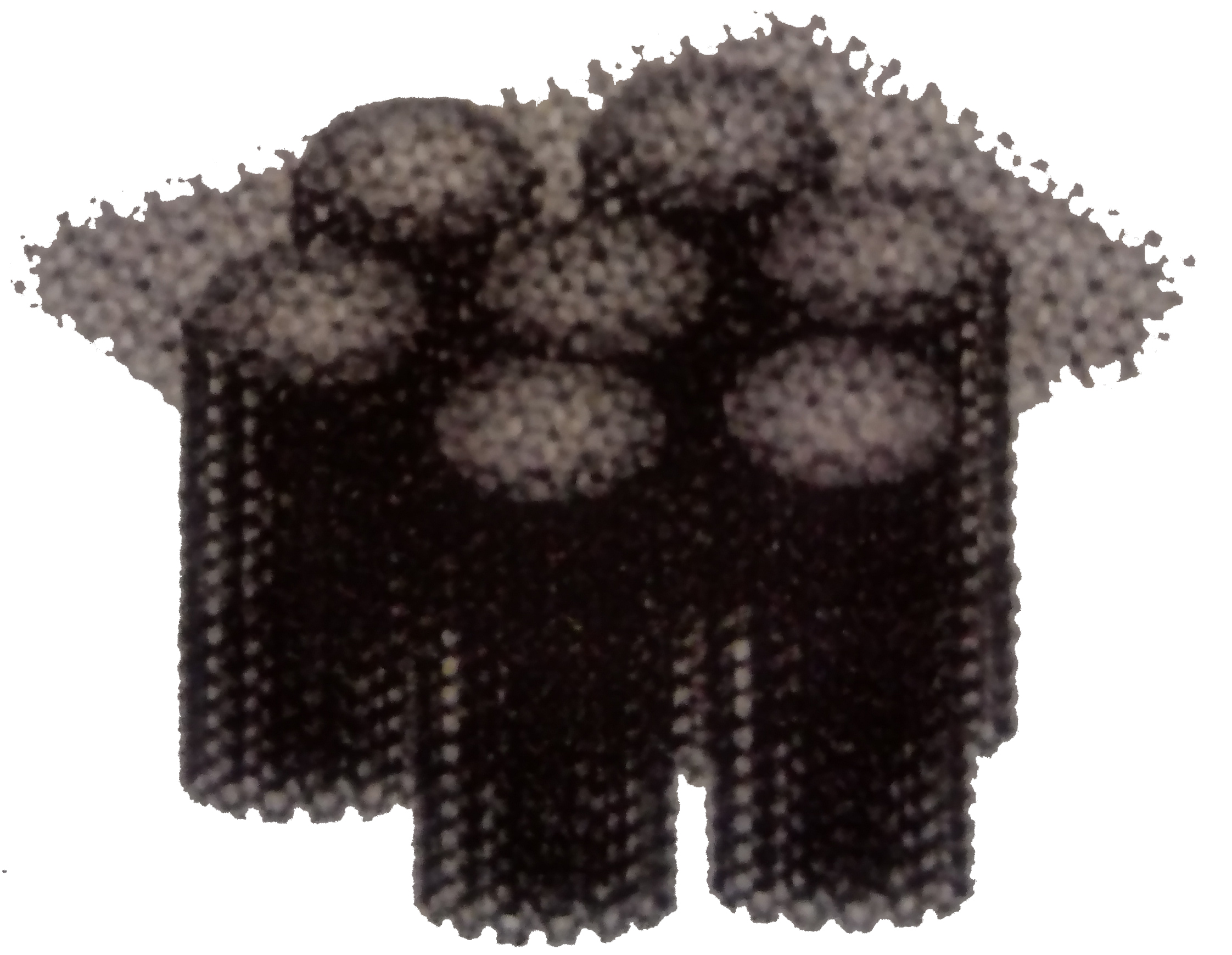 Fujitsu Laboratories has self-organized a composite structure of several to several tens of layers of graphene on multi-layered carbon nanotubes that are formed along the direction perpendicular to the substrate for carbon nanotube production. was successful.
Fujitsu Laboratories has self-organized a composite structure of several to several tens of layers of graphene on multi-layered carbon nanotubes that are formed along the direction perpendicular to the substrate for carbon nanotube production. was successful.
Research is being conducted to analyze the detailed characteristics of this composite structure of carbon nanotubes and graphene and the formation structure, and to utilize it in new fields.
CFRP artificial joints/prostheses
Research is being conducted using CFRP for artificial joints and artificial limbs. Metal is mainly used for the structural parts of conventional artificial joints. Metals have problems such as abrasion dust and osteolysis that occur when joints are repeatedly moved, bone atrophy, fatigue fracture, and reactions to metal allergies.
On the other hand, CFRP is attracting attention as an alternative material because it is lighter, stronger and has better fatigue strength than metal materials.
Compared to CFRP and metal joints, CFRP is radiolucent, allowing the use of CT and MRI. Also, since it is not metal, it does not react unnecessarily to security checks using metal detectors at airports. Furthermore, there is no problem of metal allergy.
There are still many issues to be solved in order to adopt CFRP, but in the future, artificial joints and artificial limbs made of CFRP may become mainstream.
carbon nano gauge
 A research group led by Professor Kenichiro Itami of Nagoya University has succeeded in synthesizing a basket-shaped carbon nanocompound "carbon nanogage" consisting of 120 carbon atoms and 78 hydrogen atoms for the first time in the world. The features of the carbon nanogauge are as follows:
A research group led by Professor Kenichiro Itami of Nagoya University has succeeded in synthesizing a basket-shaped carbon nanocompound "carbon nanogage" consisting of 120 carbon atoms and 78 hydrogen atoms for the first time in the world. The features of the carbon nanogauge are as follows:
i. White solid, well soluble in most organic solvents
ii. Does not decompose even at 300 degrees or more
iii. Effectively absorb light
iv. It has the characteristic of having strong blue fluorescence
.
Applications are expected to include organic EL materials, organic transistor materials, optical recording materials, high-density optical storage, fluorescence imaging of biomolecules, optical sensors of guest molecules, and precise chemical synthesis of branched carbon nanotubes. .
Introduction to Carbon List
- 1. Difference between carbon and graphite - difference between carbon and graphite
- 2. What is graphite? (Types of graphite) "Natural graphite and artificial graphite"
- 3.Manufacturing method of artificial graphite "Until the graphite block is made"
- 4.Introduction to graphite materials (differences in characteristics depending on the manufacturing method of artificial graphite materials)
- 5. Familiar carbon products, carbon products
- 6. There are graphite products in such places! ?
- 7. What will the carbon of the future look like? (New carbon/Fine carbon)
- 8.What are the properties of carbon?
Consultation and quotation for carbon products
Business hours Weekdays 8:30-17:30 (excluding weekends and holidays)








0 コメント:
コメントを投稿